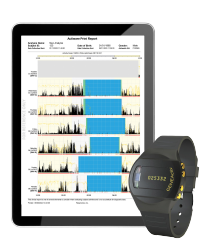
The GENEActiv wearable collects raw, unfiltered data, providing valuable real-world insights into activity, lifestyle, and sleep which help you assess interventions and treatment efficacy during your clinical trial.
Designed for long-term, continuous monitoring, capturing real-world data from participants’ daily lives outside of clinical settings, the GENEActiv has highly sensitive sensors which capture even the most subtle fine motor movements, such as tremors and scratching, along with environmental factors like light exposure and temperature.
Validated in over 1,500 publications and over 200 clinical trials across 20 therapeutic areas—including Cardiology, Dermatology (notably scratch), Neurology (with a focus on Parkinson’s Disease), Obesity, Paediatrics, and Sleep— the GENEActiv delivers real-world evidence to support your clinical trial.
Accelerate pharmaceutical drug development with GENEActiv.
Trusted by
GENEActiv Features
1500+ Publications
25+ Therapeutic Areas
200+ Clinical Trials
Digital Measures and Digital Biomarker Development
Raw, unfiltered data is an essential resource in clinical trials, future-proofing your research by enabling re-analysis with new algorithms and ensuring long-term relevance.
With the GENEActiv, you gain access to the data in an open format which can seamlessly integrate with any statistical software, giving your study access to a suite of validated digital measures. When ready, you can easily download your raw data files (.bin) using ActivInsights software, alongside AWD or CSV formats for added flexibility.
We are committed to advancing digital measures and firmly believe that open digital measures are the key to driving scientific progress. That’s why we are platform-agnostic, giving you the freedom to use your raw data files with any data processing software of your choice, ensuring maximum flexibility and control in your clinical research.’
While you can leverage this data directly, our expertise extends to processing all or part of the data for you. We will help you to select optimal measures from our catalogue in areas like circadian rhythm, energy expenditure, gait, motor function, posture, physical activity and sleep, ensuring that the data collected aligns with the study’s goals.
These digital measures serve as foundational elements for potential clinical trial endpoints, significant digital health biomarkers and clinical outcome assessments, unlocking deeper insights into disease progression, treatment efficacy, and participant lifestyle in real-world settings
Collect Real-World Data
Our GENEActiv is a ‘fit and forget’ wearable designed for long-term, continuous monitoring, lasting up to two months without needing to be charged or removed. It enables continuous, real-world data remote collection on activity, lifestyle, and sleep patterns to provide insights which support trial outcomes, and facilitate the exploration of primary, secondary, or exploratory endpoints.
Manufactured in the UK and classified as a medical device with FDA 510(k) exemption status, it has been validated in over 1,500 peer-reviewed publications and more than 200 clinical trials, achieving adherence rates of over 90%.
The GENEActiv’s ease of use and high adherence simultaneously reduces the burden on trial site while collecting significantly more data than traditional methods, capturing objective insights in participants’ natural environments—data that was previously challenging to obtain.
Deployed in over 40 countries and used in studies with up to 10,000 participants supporting hybrid and decentralised trial models, making the GENEActiv ideal for capturing objective, real-world data at scale.
Conducting clinical research at scale in real-world settings has never been easier.
Essential GENEActiv Accessories
When you buy a GENEActiv device, you will need to purchase a USB cradle.
The charging cradle not only powers the devices but also serves as the tool for programming them and downloading your data.
Each charging cradle can hold up to 4 devices. Our team will be able to advise on how many cradles would be recommended for your study.
Your devices will be supplied with black PU resin wrist straps as standard, however, we cater for studies for children, other wear sites and ruggedised applications.

Regulatory Compliance
The GENEActiv has FDA 510(k) exemption status.
GENEActiv falls under the classification of Class I Non-Sterile Medical Devices, under the Medical Device Directive 93/42/EEC.In accordance with transitional provisions outlined in Regulation 2023/607, amending Regulations (EU) 2017/745 and (EU) 2017/746, we will be transitioning to compliance with the EU Medical Device Regulation (EU MDR).