News &
Insights
13 October 2022
Digital Biomarkers: The Future is Here!
Digital Biomarkers: The Future is Here! By Bill Hogan, CEO A quick visit to the […]
Share This Post, Choose Your Platform!

Digital Biomarkers: The Future is Here!
By Bill Hogan, CEO
A quick visit to the US National Library of Medicine and specifically ClinicalTrials.gov and you can read the following…
“In a clinical trial, participants receive specific interventions according to the research plan or protocol created by the research team. These interventions may be medical products, such as drugs or devices; procedures; or changes to participants’ behaviour such as diet. Clinical trials may compare a new medical approach to a standard one that is already available, to a placebo that contains no active ingredients, or to no intervention. Some clinical trials compare interventions that are already available to each other.
When a new product or approach is being studied, it is not usually known whether it will be helpful, harmful, or no different than available alternatives. Investigators try to determine the safety and efficacy of the intervention by measuring certain outcomes in the participants.”
So far, so good! Have you ever wondered though how detailed, thorough, or relevant is the data generated as described above. Certainly, the recent global pandemic has seen a very significant shift towards decentralised and virtual clinical trials. Under-pinning these shifts has been the proliferation of hardware and software that enables data collection remotely.
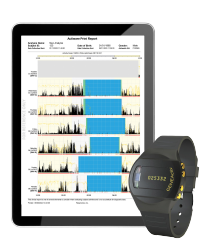
Frequent, objective and sensitive assessments are critical to measuring health trends and determine the efficacy of new therapeutics. Traditionally, intermittent clinical observations at long intervals were the standard accepted practice. Throughout the pandemic we saw increased validation and verification of continuous monitoring. Wearable accelerometers can continuously capture data to assess health and today we are seeing the rise of digital biomarkers as a result.
Real-world Raw Data
Real-world data derived from objective raw data is key to this continued development. Not all devices will deliver raw data! The ability to realise primary and secondary digital clinical end-points is built upon the availability of raw data and ability to translate the data analytics into digital clinical end points and health biomarkers.
The Digital Medicine Society (DiMe) is a global non-profit organisation and a base for the global digital medicine community. Their aim is to drive scientific progress and broad acceptance in digital medicine to enhance public health. We are about to work closely with DiMe on a project to define an optimized set of core digital clinical measures that address patient, care-partner, and clinical unmet need for aspects of health that span multiple therapeutic areas in physical activity – a domain where digital clinical measurement capabilities are already well advanced.
We are very much looking forward to the next phase of our collaboration with DiMe.